NIH Biosafety Level I
Certification 2021






ABRI (Allied Biomedical Research Institute) is an independent and neutral source of bio-products which is of an eminent service to its customers....
ABRI engages in the collection of biological materials, such as blood, tissue, and other samples.
ABRI specializes in conducting clinical trials, which are research studies performed on people to evaluate medical, surgical, or behavioral...
Phase 1 clinical trials are the first stage of testing in human subjects and are a critical component of the drug development process. At Allied...
ABRI is an independent and neutral source of bio-products, which is of an eminent service to its customers. When a customer has the need for a collection of rare, highly characterized biological samples, ABRI is the one provider that is able to constantly deliver high quality samples in a timely and efficient manner and within full regulatory compliance. ABRI excels in its understanding of the regulatory environment and project management capacities to enable the commercialization of regulated products. ABRI customers, most of them are involved in time-reliable product for development; rely on ABRI to expedite their time to market.
ABRI operates and holds a worldwide permit with the Center of Disease Control and Prevention (CDC), register with the Food and Drug Administration (FDA) and functions with an Internal Review Board (IRB) to meet all standards and procedures. For further information on the ABRI's facilities and operations, please feel free to contact us


Certification 2021
Re-Certification 2022
2008 – 2022
Ongoing
At ABRI, we are actively looking for individuals just like you to help answer important questions about a variety of health conditions.
Clinical Research participants help the discovery and implementation of important medical innovations to include genetics, drugs, clinical techniques, and diagnostic tools.

A dedicated ABRI staff member will review the different diagnosis and match each individual potential participant to a study.
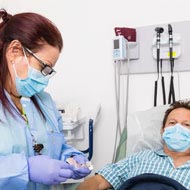
ABRI physicians and clinical team will monitor your health at all times.

Patient will receive information when a study is getting to the end. Several studies offer Open Label Extensions which mean, the participant will continue to receive the actual medication for an extended period of time.
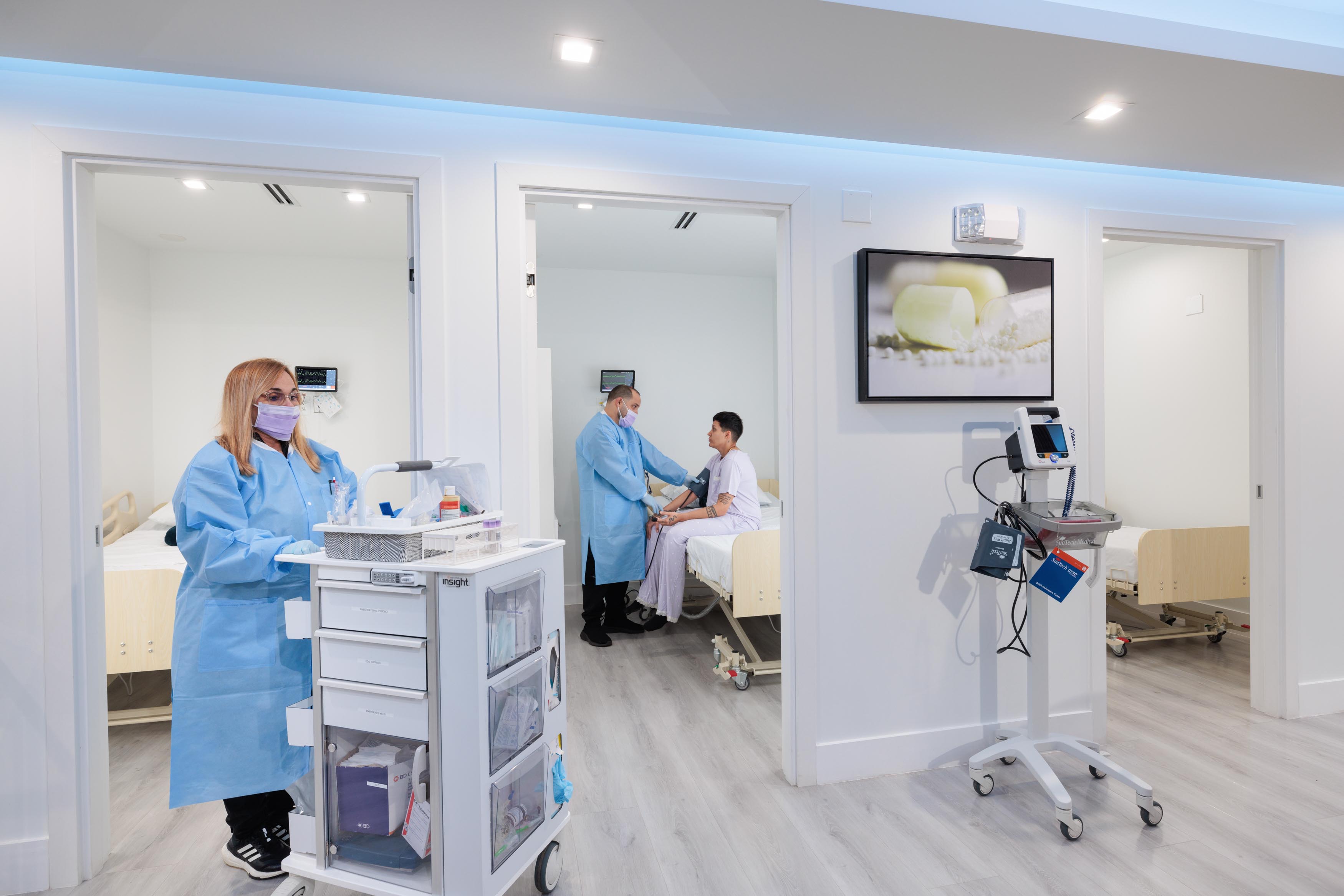
ABRI Phase 1 Center is a state-of-the-art facility, flexible and adaptable for single or multi clinical trials. For long or short stays, our well-appointed clinical units have all the capabilities to conduct clinical trials in a number of therapeutic areas.
We are expertly staffed, with dedicated pharmacy, laboratory, and with capability for specialized assessments.
Our center has the capability to conduct multi-specialty trials, under the supervision of a professional and experienced team comprise of supervisors, doctors, research coordinators, etc.
At our center, every patient will have his/her own private hospital-like rooms.
Our center is fully equipped with a modern cardiac and respiratory telemetry, a way to continuously monitor a patient's heart's electrical activity, respiration and other vital signs. We prioritize patient monitoring and safety, including dedicated examination rooms, a nursing station, a drug administration area, a waiting room, a reception/entertainment area, secure drug storage, and office space for research staff.
ABRI also has an outpatient clinic for phases II — IV adjacent to the phase I. The outpatient clinic has several treatment rooms, psychometric quiet rooms, pharmacy, and a biosafety level II laboratory.
During these phases, patient will have the chance of participating in medium to large studies that will allow the pharmaceutical industry to compare the safety and effectiveness of the new treatments against the current standard treatments. Participants will also benefit from clinic doctor's visits, therapies and stipend for their participation and time.
An ABRI professional will gladly provide more information about our phase II-IV outpatient clinic services and studies.

Allied Biomedical Research Institute (ABRI) is a global integrated biotechnology and clinical research company with its headquarters in the United States and sites in South Florida (US), Santo Domingo (Dominican Republic), Guayaquil (Ecuador), Ciudad Bolivar, Maiquetilla, and Puerto Ordaz (Venezuela) and Ahmedabad (India).
We are here for the individuals, supporting their quest of healthy lives. This has been our way for over period close to a decade. ABRI is passionately and thoughtfully rendering science into lasting supports to health.
Scientific Excellence and Patient Well-being. ABRI is committed to achieving scientific excellence in all its endeavors. This means conducting high-quality research that adheres to rigorous scientific standards. Additionally, ABRI prioritizes patient well-being, ensuring that the rights, safety, and health of participants are protected throughout the research process.
Future of Medicine. By conducting innovative research and clinical trials, ABRI aims to contribute significantly to the future of medicine. Their work helps pave the way for new treatments and therapies that can improve patient outcomes and advance healthcare.
Reliable Center of Medical Research and Innovation.
ABRI positions itself as a reliable and leading center for medical research and innovation. Based in Miami, Florida, ABRI is at the forefront of biomedical research, conducting groundbreaking clinical trials across a wide range of therapeutic areas. Their dedication to scientific excellence and patient well-being drives their mission to shape the future of medicine.
Allied Biomedical Research Institute (ABRI) is a state-of-the-art standalone U.S.-based clinical research organization with subsidiary centers in South America, Africa, and other regions. ABRI conducts clinical trials from Phase I to Phase IV across various therapeutic areas and collects biological materials to support clinical research and maintain an inventory of bio-products. Dedicated to scientific excellence and patient well-being, ABRI aims to be a reliable center of medical research and innovation, contributing to the future of medicine through groundbreaking clinical trials and advanced research.
Our research staff has a combined 20+ years’ experience conducting Phase I-IV clinical studies and our success in high enrollment is contributed to a full-time experienced recruitment and marketing team with a dedicated 24-hour call center.

Providing support to pharmaceutical sponsors, clinical research organizations for the conduction of Clinical Trials throughout multiple geographies.
Providing Audit support during state and/or federal inspections primarily FDA and CDC.
Consulting Service to Evaluate Protocols, Investigator Brochure, Electronic Case Forms, IWRS/IVRS.
ABRI network will make available a variety of practice specialties and subspecialties for the conduction of clinical trials though out multiple geographies.


ABRI collects biological materials to meet the clinical trial management solutions and for its own inventory of bio-products, that is provided to customers, who are involved in research, studies and development.
ABRI is also an independent and neutral source of bio-products, offering a range of services, products and technologies for the selection, development and manufacture of biopharmaceuticals, products used for laboratory validation and costumed panels.
ABRI es un instituto de investigación biomédica que se especializa en estudios clínicos avanzados.
La investigación clínica implica estudios realizados con voluntarios humanos para evaluar intervenciones médicas, quirúrgicas o de comportamiento. Su objetivo es determinar la seguridad y la eficacia de nuevos tratamientos, medicamentos, dispositivos o procedimientos.
La investigación clínica ayuda a avanzar en el conocimiento médico, mejorar la atención al paciente y desarrollar nuevos tratamientos para enfermedades. Garantiza que las terapias sean seguras y efectivas antes de su uso generalizado.
Cada ensayo clínico tiene criterios de elegibilidad específicos basados en factores como la edad, el género, la historia clínica, el estado de salud actual y la enfermedad en estudio. Estos criterios garantizan la seguridad del participante y la fiabilidad de los resultados del estudio.
Fase I: Prueba la seguridad de un tratamiento en un grupo pequeño de personas. Fase II: Evalúa la efectividad y profundiza en la evaluación de la seguridad. Fase III: Confirma la efectividad, supervisa los efectos secundarios y compara con tratamientos estándar. Fase IV: Se realiza después de la aprobación de la FDA para recopilar información adicional sobre efectos a largo plazo.
Los participantes pueden tener acceso a nuevos tratamientos antes de que estén ampliamente disponibles, recibir atención médica experta y contribuir al avance de la ciencia médica.
Las políticas de compensación varían según el estudio. Algunos ensayos ofrecen compensación económica por el tiempo y los gastos de viaje. Esta información se proporcionará antes de que decidas participar.
Sí, la participación es completamente voluntaria. Puedes retirarte en cualquier momento sin penalización ni pérdida de beneficios.
Toda la información personal y médica se mantiene confidencial y solo se comparte con el personal autorizado que participa en el estudio. Por lo general, los datos se anonimizan cuando se informa.
Los participantes pasan por un proceso de evaluación para determinar la elegibilidad, seguido de la inscripción si cumplen los requisitos. El equipo del estudio supervisa de cerca a los participantes durante todo el ensayo, lo que puede incluir pruebas, tratamientos o cuestionarios.
La duración varía según el diseño del estudio. Algunos ensayos duran semanas, mientras que otros pueden prolongarse durante meses o años.
Un placebo es una sustancia inactiva diseñada para imitar el tratamiento que se está probando. Algunos estudios utilizan placebos para comparar los resultados, pero esto se explicará durante el proceso de consentimiento informado.
El consentimiento informado es un proceso en el que se proporciona a los participantes información detallada sobre el estudio, incluido su propósito, procedimientos, riesgos y beneficios, para que puedan tomar una decisión informada sobre su participación.
Después de que concluye un ensayo, los investigadores analizan los datos para determinar la seguridad y la efectividad del tratamiento. Los resultados pueden publicarse en revistas científicas y los tratamientos exitosos pueden pasar a la aprobación regulatoria.